Zehra Nur KoyuncuT., Nida Dereli ÇalışkanE.
With the advancement of technology, there has been a tremendous expansion of scientific knowledge. This flow of information has led to the emergence of interdisciplinary fields such as “Synthetic Biology (SB)”. This integrates concepts from biological sciences, engineering, and computational sciences. SB is an approach that aims to integrate the principles of biological systems with concepts from other disciplines. In SB, the goal is to redesign, reprogram, and create new biological systems by manipulating the natural biological systems found within genetic circuits. Genetic circuits are functional combinations of basic biological entities (DNA, RNA, proteins) created using typical design, build, test, and learn (DBTL) cycles1-4. SB is a multidisciplinary approach that utilizes various disciplines to construct new organisms within the human body and produce useful products in energy, the environment, agriculture, and medicine5 (Figure 1)6. This review will delve into the fundamental components of SB and discuss its potential applications, highlighting recent advancements in the field.
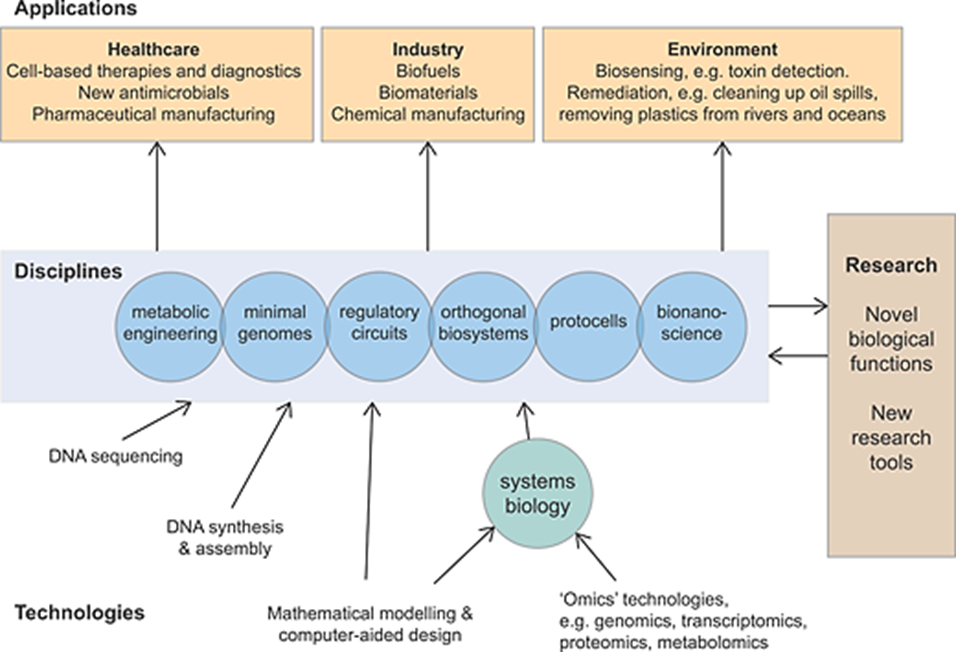
Figure 1. Overview of synthetic biology6.
- Synthetic Biology Approaches
SB is based on two different approaches. The first one is the “top-down” approach, where cells are modified using molecular biology and metabolic/genetic engineering techniques. An alternative approach involves the construction of cell-like structures called artificial cells (proto-cells or synthetic cells) from non-living building blocks, which is referred to as the “bottom-up” approach7. The top-down approach aims to derive synthetic cells from biologically modified cells, for example, by manipulating genes and protein content. These synthetic cells are typically alive but still closely related to their biological ancestors. A well-known example of SB through the top-down approach is the minimal genome projects, which aim to identify the core genes of a specific species. Such genomes have been successfully initiated within living cells8, 9. On the other hand, the bottom-up approach in cell design starts with inanimate matter. A synthetic cell is a compartment of cellular size. Cell-like functionality is achieved by reorganizing modules made from natural or artificial molecular building blocks. The complexity of a synthetic cell is gradually increased by incorporating more and more components. This strategy has been successfully used to uncover the biological role of individual proteins10, 11.
2. Applications of Synthetic Biology
2.1. Genetic Manipulation
Genetic manipulation is the most well-known application of SB. In this approach, the two main targets are the genome and extrachromosomal plasmids in cells. Plasmids are preferred as transferable elements for basic applications. As genomic manipulation tools have grown and become more user-friendly (e.g., CRISPR), genetic manipulations in SB are increasingly performed at the genome level12.
2.2. Synthetic Microbial Consortia
The scientific community has become interested in microbial consortia (or communities) of microbes because of their diverse natural distribution and crucial roles in biochemical cycles. The competitive interactions between species and strains within microbial consortia and their cooperative approaches to survival enable these communities to form more complex phenotypes13. Synthetic microbial consortia refer to simple microbial communities designed with a defined composition of two or more (typically 2-3) species/strains and are tailored for biotechnological applications. Synthetic biologists apply design principles such as top-down and bottom-up approaches to create synthetic microbial consortia with numerous real-life applications in healthcare, disease prevention, and environmental remediation14-16. Creating synthetic microbial consortia relies on fundamental cell-cell communication mechanisms, one of which is quorum sensing (QS). QS, which offers dynamic solutions for metabolic engineering, involves signal molecules known as autoinducers that are released from the intracellular to the extracellular environment. When a specific threshold (often at high cell density) is reached, these molecules trigger or coordinate the expression of target genes17. In addition, in the context of consortium studies, the CRISPR/Cas9 gene editing system, single-cell technologies, and tools from systems biology are also used for DNA and genetic circuit assembly13, 18, 19.
2.3. Sustainable Production and Climate Crisis
The production of thousands of chemicals derived from fossil sources, such as fuels, plastics, and industrial chemicals like petroleum and natural gas, has become a significant target for reducing global greenhouse gas emissions in the face of the current climate crisis. Sustainable production of gases with industrial value, such as acetone and isopropanol (IPA), through fermentation, is crucial for material engineering. The current sugar fermentation method presents sustainability challenges. Modified bacteria through SB offer an environmentally friendly model where waste gases, including carbon dioxide, can be converted into valuable chemicals, such as acetone and IPA (isopropyl alcohol), on a large scale, contributing to the energy cycle20.
2.4. Agriculture and Nutrition
Agriculture, another industry sector negatively impacted by population growth and the climate crisis, requires alternative solutions due to inadequate technologies to meet increasing demands and the declining productivity of agricultural products due to the use of harmful chemicals like pesticides and herbicides. Therefore, the Green Revolution, aiming to mass apply and develop synthetic and natural fertilizers (phosphorus, nitrogen, and potassium), as well as cultivation strategies to maximize plant architecture and harvest, has been an approach resulting in higher yields21. Additionally, producing food-based cell factories or biofortification of foods through SB, enabling bio-synthesis, has emerged as an essential area of study for sustainable food supply worldwide22, 23.
2.5. Industrial Biotechnology
Approximately 20% of SB start-ups focus on industrial biotechnology objectives. Industrial biotechnology (IB), defined as the use of enzymes and microorganisms for the production of bio-based products in sectors such as food, chemicals, feed, paper, detergents, bioenergy (biofuels), and textiles, primarily involves maximizing and optimizing the biochemical pathways used in production through working with natural systems24. A Biological Foundry (BioFAB) refers to an integrated molecular biology facility with high-throughput processing and analytical equipment, the software, personnel, and data management systems required to operate this equipment, and broader biological foundry capabilities. It combines automation engineering with SB to create new high-efficiency biological solutions that assist in developing and empowering a DBTL approach to biological engineering while offering a suitable bioeconomy model for industrial advancement (Figure 2)25.
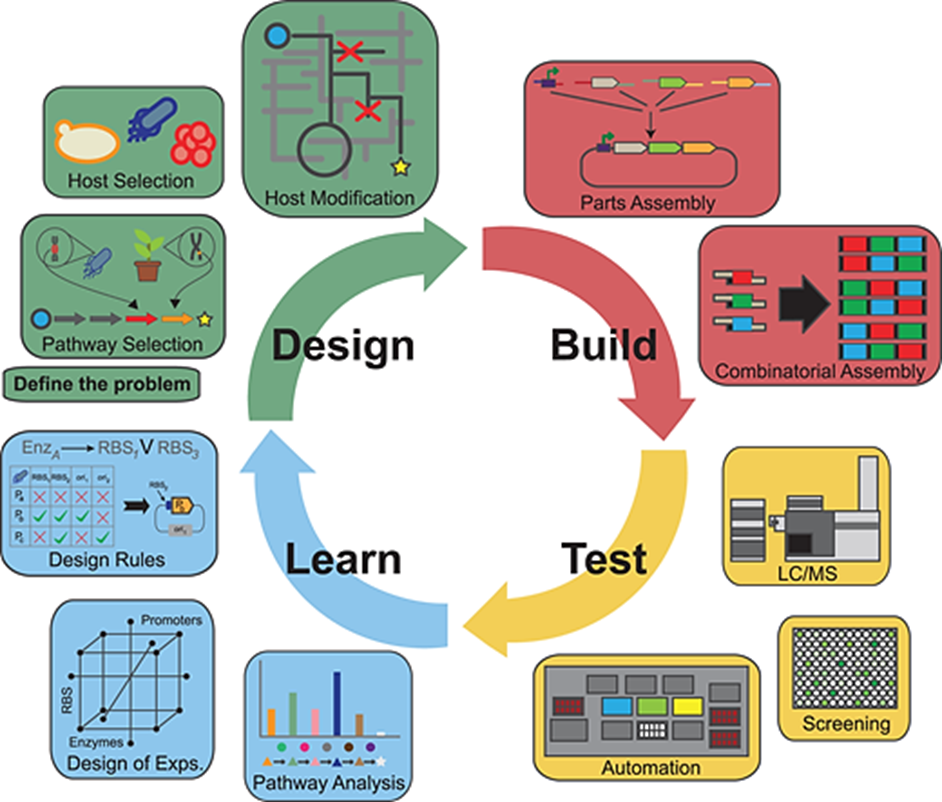
Figure 2. DBTL diagram25. The cycle depicted in the figure is iterated through the stages of design, build, test, and learning, which involve the computational design of genetic regions, circuits, and regulatory and metabolic processes.
2.6. Metabolic Engineering
Metabolic engineering is an approach that allows for developing microbial strains that efficiently produce chemicals and materials. Still, it requires considerable time, effort, and cost to make the strains industrially competitive26. The integration of SB into this approach has facilitated the design of biosynthetic microorganisms and the production of valuable products from them27. SB has enabled the development of artificial regulatory circuits for applications in metabolic engineering28.
- Tissue Engineering and Living Biomaterial Production
The principles of tissue engineering are based on repairing and replacing damaged or lost tissues. While advances in biomaterial synthesis and stem cell technologies contribute to in vitro applications in this field, the manipulation of tissue-specific cells faces challenges that can be addressed with the principles of SB29. Genetic circuits created with SB technologies, along with the control of intracellular signaling, offer an alternative potential for designing next-generation biomaterials by enabling the manageability of the extracellular environment30. The collaboration between SB and materials science in the biologicalization of materials involves the reprogramming of living systems such as microorganisms to function as dynamic and responsive materials, using sensor-regulated genetic circuits, thus defining the field of Materials Synthetic Biology31-33.
- Disease Diagnosis and Drug Discovery
SB, combined with simultaneous advancements in protein engineering, allows for the design of cells that can activate signalling pathways in living systems using synthetic receptors. These systems enable the construction of theragnostic cells that can serve as both diagnostic tools and therapeutic delivery systems34. SB technologies have facilitated the development of several innovative approaches, primarily in the preclinical stage, for the diagnosis of non-infectious diseases such as enzymatic functions, infectious diseases, and coronary artery disease (e.g., paper-based RNA switch sensors, CRISPR-based diagnostics, etc.)35. The integration of sensitive nanoparticles or molecular probes with SB principles is expected to open up new diagnostic pathways specific to many diseases, including cancer. Studies are being conducted to detect the altered transcriptional state found in cancer cells by designing synthetic circuits based on transcriptional control, incorporating molecular components such as synthetic promoters with enhanced cell-state specificity (SPECS)36.
Advancements in SB enable the reprogramming of synthetic cells as intelligent agents to target tumors and achieve controlled release of anti-cancer drugs37. Research on designing SB-based living biotherapeutics is increasing, particularly in various human pathologies such as inflammatory and metabolic diseases38. SB has already made a significant impact on the development and therapeutic production of pharmaceuticals throughout the drug discovery process. Thanks to, The organization of synthetic DNA, along with the increased understanding of gene regulation and genome details, is expected to form the basis for current successes and potential future applications, accompanied by reduced costs. Rapid advancements are being made in fields such as immunotherapy, cell therapy, and biotherapeutics, including vaccines, thanks to SB35, 39.
- Biosensors
Cell-based biosensors utilize the natural ability of a cell to perceive and respond to its environment by repositioning its detection mechanisms in new genetic contexts. They aim to produce cells capable of detecting specific target molecules and generating a response to them. Cell-based biosensors have gained significant interest as an alternative detection method due to various advantages such as detection sensitivity, cost-effectiveness, portability, and the absence of specialized equipment and trained personnel compared to traditional methods. The use of directed evolution is highly preferred for constructing or improving SB approaches in biosensor structures40. Recombinases provide the opening/closing function in logic gates in the field of SB, enabling the design of genetic circuits that can compute and remember. Recombinase-based whole-cell biosensors are developed for the purpose of detecting environmental signals41,42.
- Protein Engineering
Protein-protein interactions (PPIs) govern many cellular functions in living systems. Designing new PPIs to elucidate molecular interactions poses a fundamental challenge in protein engineering43. By leveraging SB technologies, reversible PPI-based protein circuits or synthetic biological toggle switches that respond within seconds can be designed, providing insights into new biological interactions and enabling the construction of cellular functional networks44, 45.
- Beyond the Laboratory: Applications of Synthetic Biology
Most scientific advancements are conducted in well-controlled laboratory settings and cannot be immediately translated into “off-laboratory” applications. The use of SB tools in such applications is considered a potential problem solver for producing various compounds, therapeutics, and materials in unconventional remote environments, as well as meeting the needs of the drug, fuel, or general resource storage in military and space missions. It is believed that SB applications capable of going beyond the confines of a laboratory can provide lasting improvements for human health and environmental applications46.
- Synthetic Cells and Biomedical Applications
Cells have the ability to come together as the building blocks of everything around us, forming tissues and other complex structures. This has led to the development of synthetic cells (SCs) that can mimic live cells, particularly for clinical applications. SCs can mimic live cells while providing advantages for cellular stability studies under conditions incompatible with life. Building functional synthetic cells from the bottom up remains an ongoing effort for scientists worldwide, although there are various limitations47. Two recently published studies have shed light on the path to life-like human-made systems in SCs. In the first study that explains the integration of cell division mechanisms into SC, a successful reconstruction of active bacterial division machinery mimicking the mechanism in Escherichia coli was achieved by integrating the relevant proteins onto a synthetic membrane called Janus dendrimers. This study represents a step towards designing synthetic entities that can leverage mechanisms provided by nature48. In the second study, functional DNA-based cell skeletons were incorporated into cell-sized compartments, allowing them to perform functions such as molecular transport or assembly and disassembly on specific triggers49. SCs are considered potential structures for various biomedical applications, including therapeutic discovery, nanomaterial synthesis, photochemical control of intracellular functions, and artificial enzyme production47, 50.
Consequently, SB, an approach to integrating biology with more engineering applications, aims to design existing or novel systems by creating various biological components within a specific standardization framework. While the development of application-oriented tools and interdisciplinary approaches has led to advancements in the field of SB, the lifelong developmental capacity of living systems poses a major challenge in the synthetic design of biological systems. However, it has contributed to enhancing our understanding of fundamental cellular processes through the DBTL method, leading to a wide range of diverse applications in academia, industry, and the clinic. The rapid advancements in SB hold the potential to improve the quality of human life, but they also come with certain risks. While synthetic biology plays a key role in realizing futuristic ideas such as designing functional cells, genetic manipulation, and more, addressing ethical concerns is important to prevent the misuse of such technologies and similar ones.
References
- MacDonald, I. C., Seamons, T. R., Emmons, J. C., Javdan, S. B., & Deans, T. L. (2021). Enhanced regulation of prokaryotic gene expression by a eukaryotic transcriptional activator. Nature Communications, 12(1). https://doi.org/10.1038/S41467-021-24434-9
- Saltepe, B., Kehribar, E. Ş., Su Yirmibeşoǧlu, S. S., & Şafak Şeker, U. Ö. (2018). Cellular Biosensors with Engineered Genetic Circuits. ACS Sensors, 3(1), 13–26. https://doi.org/10.1021/acssensors.7b00728
- Xiang, Y., Dalchau, N., & Wang, B. (2018). Scaling up genetic circuit design for cellular computing: advances and prospects. Natural Computing 2018 17:4, 17(4), 833–853. https://doi.org/10.1007/S11047-018-9715-9
- Carbonell, P., Jervis, A. J., Robinson, C. J., Yan, C., Dunstan, M., Swainston, N., Vinaixa, M., Hollywood, K. A., Currin, A., Rattray, N. J. W., Taylor, S., Spiess, R., Sung, R., Williams, A. R., Fellows, D., Stanford, N. J., Mulherin, P., Le Feuvre, R., Barran, P., … Scrutton, N. S. (2018). An automated Design-Build-Test-Learn pipeline for enhanced microbial production of fine chemicals. Communications Biology 2018 1:1, 1(1), 1–10. https://doi.org/10.1038/s42003-018-0076-9
- Nxumalo, Z., & Thimiri Govinda Raj, D. B. (2020). Application and challenges of synthetic biology. Advances in Synthetic Biology, 307–320. https://doi.org/10.1007/978-981-15-0081-7_18
- Garner, K. L. (2021). Principles of synthetic biology. Essays in Biochemistry, 65(5), 791. https://doi.org/10.1042/EBC20200059
- Ausländer, S., Ausländer, D., & Fussenegger, M. (2017). Synthetic Biology-The Synthesis of Biology. Angewandte Chemie (International Ed. in English), 56(23), 6396–6419. https://doi.org/10.1002/ANIE.201609229
- Lachance, J. C., Rodrigue, S., & Palsson, B. O. (2019). Minimal cells, maximal knowledge. ELife, 8. https://doi.org/10.7554/ELIFE.45379
- Simons, M. (2021). Synthetic biology as a technoscience: The case of minimal genomes and essential genes. Studies in History and Philosophy of Science Part A, 85, 127–136. https://doi.org/10.1016/J.SHPSA.2020.09.012
- Damiano, L., & Stano, P. (2020). On the “Life-Likeness” of Synthetic Cells. Frontiers in Bioengineering and Biotechnology, 8, 953. https://doi.org/10.3389/FBIOE.2020.00953
- Guindani, C., da Silva, L. C., Cao, S., Ivanov, T., & Landfester, K. (2022). Synthetic Cells: From Simple Bio‐Inspired Modules to Sophisticated Integrated Systems. Angewandte Chemie (International Ed. in English), 61(16). https://doi.org/10.1002/ANIE.202110855
- Hanczyc, M. M. (2020). Engineering Life: A Review of Synthetic Biology. Artificial Life, 26(2), 260–273. https://doi.org/10.1162/ARTL_A_00318
- Alnahhas, R. N., Sadeghpour, M., Chen, Y., Frey, A. A., Ott, W., Josić, K., & Bennett, M. R. (2020). Majority sensing in synthetic microbial consortia. Nature Communications 2020 11:1, 11(1), 1–10. https://doi.org/10.1038/s41467-020-17475-z
- Liang, Y., Ma, A., & Zhuang, G. (2022). Construction of Environmental Synthetic Microbial Consortia: Based on Engineering and Ecological Principles. Frontiers in Microbiology, 13, 437. https://doi.org/10.3389/fmicb.2022.829717
- Amor, D. R., & Bello, M. D. (2019). Bottom-Up Approaches to Synthetic Cooperation in Microbial Communities. Life 2019, Vol. 9, Page 22, 9(1), 22. https://doi.org/10.3390/LIFE9010022
- Danchin, A. (2022). In vivo, in vitro and in silico: an open space for the development of microbe‐based applications of synthetic biology. Microbial Biotechnology, 15(1), 42. https://doi.org/10.1111/1751-7915.13937
- Stephens, K., & Bentley, W. E. (2020). Synthetic Biology for Manipulating Quorum Sensing in Microbial Consortia. Trends in Microbiology, 28(8), 633–643. https://doi.org/10.1016/J.TIM.2020.03.009
- McCarty, N. S., & Ledesma-Amaro, R. (2019). Synthetic Biology Tools to Engineer Microbial Communities for Biotechnology. Trends in Biotechnology, 37(2), 181. https://doi.org/10.1016/J.TIBTECH.2018.11.002
- Ben Said, S., Tecon, R., Borer, B., & Or, D. (2020). The engineering of spatially linked microbial consortia – potential and perspectives. Current Opinion in Biotechnology, 62, 137. https://doi.org/10.1016/J.COPBIO.2019.09.015
- Liew, F. E., Nogle, R., Abdalla, T., Rasor, B. J., Canter, C., Jensen, R. O., Wang, L., Strutz, J., Chirania, P., De Tissera, S., Mueller, A. P., Ruan, Z., Gao, A., Tran, L., Engle, N. L., Bromley, J. C., Daniell, J., Conrado, R., Tschaplinski, T. J., … Köpke, M. (2022). Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale. Nature Biotechnology 2022 40:3, 40(3), 335–344. https://doi.org/10.1038/s41587-021-01195-w
- Roell, M. S., & Zurbriggen, M. D. (2020). The impact of synthetic biology for future agriculture and nutrition. Current Opinion in Biotechnology, 61, 102–109. https://doi.org/10.1016/J.COPBIO.2019.10.004
- Lv, X., Wu, Y., Gong, M., Deng, J., Gu, Y., Liu, Y., Li, J., Du, G., Ledesma-Amaro, R., Liu, L., & Chen, J. (2021). Synthetic biology for future food: Research progress and future directions. Future Foods, 3, 100025. https://doi.org/10.1016/J.FUFO.2021.100025
- Pouvreau, B., Vanhercke, T., & Singh, S. (2018). From plant metabolic engineering to plant synthetic biology: The evolution of the design/build/test/learn cycle. Plant Science, 273, 3–12. https://doi.org/10.1016/J.PLANTSCI.2018.03.035
- Clarke, L., & Kitney, R. (2020). Developing synthetic biology for industrial biotechnology applications. Biochemical Society Transactions, 48(1), 113–122. https://doi.org/10.1042/BST20190349
- Freemont, P. S. (2019). Synthetic biology industry: data-driven design is creating new opportunities in biotechnology. Emerging Topics in Life Sciences, 3(5), 651. https://doi.org/10.1042/ETLS20190040
- Xu, X., Liu, Y., Du, G., & Liu, L. (2020). Systems biology, synthetic biology, and metabolic engineering. Systems and Synthetic Metabolic Engineering, 1–31. https://doi.org/10.1016/B978-0-12-821753-5.00001-0
- Shomar, H., & Bokinsky, G. (2021). Towards a Synthetic Biology Toolset for Metallocluster Enzymes in Biosynthetic Pathways: What We Know and What We Need. Molecules, 26(22). https://doi.org/10.3390/MOLECULES26226930
- Peng, B., Bandari, N. C., Lu, Z., Howard, C. B., Scott, C., Trau, M., Dumsday, G., & Vickers, C. E. (2022). Engineering eukaryote-like regulatory circuits to expand artificial control mechanisms for metabolic engineering in Saccharomyces cerevisiae. Communications Biology, 5(1). https://doi.org/10.1038/S42003-022-03070-Z
- Hoffman, T., Antovski, P., Tebon, P., Xu, C., Ashammakhi, N., Ahadian, S., Morsut, L., & Khademhosseini, A. (2020). Synthetic Biology and Tissue Engineering: Toward Fabrication of Complex and Smart Cellular Constructs. Advanced Functional Materials, 30(26), 1909882. https://doi.org/10.1002/ADFM.201909882
- Weisenberger, M. S., & Deans, T. L. (2018). Bottom-up approaches in synthetic biology and biomaterials for tissue engineering applications. Journal of Industrial Microbiology and Biotechnology, 45(7), 599–614. https://doi.org/10.1007/S10295-018-2027-3
- Tang, T. C., An, B., Huang, Y., Vasikaran, S., Wang, Y., Jiang, X., Lu, T. K., & Zhong, C. (2020). Materials design by synthetic biology. Nature Reviews Materials 2020 6:4, 6(4), 332–350. https://doi.org/10.1038/s41578-020-00265-w
- Burgos-Morales, O., Gueye, M., Lacombe, L., Nowak, C., Schmachtenberg, R., Hörner, M., Jerez-Longres, C., Mohsenin, H., Wagner, H. J., & Weber, W. (2021). Synthetic biology as driver for the biologization of materials sciences. Materials Today Bio, 11, 100115. https://doi.org/10.1016/J.MTBIO.2021.100115
- Özkul, G., Yavuz, M., Haciosmanoğlu, N., Kirpat, B. M., & Şeker, U. Ö. Ş. (2022). Design and applications of self-assembled soft living materials using synthetic biology. New Frontiers and Applications of Synthetic Biology, 361–372. https://doi.org/10.1016/B978-0-12-824469-2.00006-3
- McNerney, M. P., Doiron, K. E., Ng, T. L., Chang, T. Z., & Silver, P. A. (2021). Theranostic cells: emerging clinical applications of synthetic biology. Nature Reviews Genetics 2021 22:11, 22(11), 730–746. https://doi.org/10.1038/s41576-021-00383-3
- Tan, X., Letendre, J. H., Collins, J. J., & Wong, W. W. (2021). Synthetic biology in the clinic: engineering vaccines, diagnostics, and therapeutics. Cell, 184(4), 881–898. https://doi.org/10.1016/J.CELL.2021.01.017
- He, J., Nissim, L., Soleimany, A. P., Binder-Nissim, A., Fleming, H. E., Lu, T. K., & Bhatia, S. N. (2021). Synthetic Circuit-Driven Expression of Heterologous Enzymes for Disease Detection. ACS Synthetic Biology. https://doi.org/10.1021/acssynbio.1c00133
- Lim, B., Yin, Y., Ye, H., Cui, Z., Papachristodoulou, A., & Huang, W. E. (2022). Reprogramming Synthetic Cells for Targeted Cancer Therapy. ACS Synthetic Biology, 11(3), 1349–1360. https://doi.org/10.1021/acssynbio.1c00631
- Ozdemir, T., Fedorec, A. J. H., Danino, T., & Barnes, C. P. (2018). Synthetic Biology and Engineered Live Biotherapeutics: Toward Increasing System Complexity. Cell Systems, 7(1), 5–16. https://doi.org/10.1016/J.CELS.2018.06.008
- David, F., Davis, A. M., Gossing, M., Hayes, M. A., Romero, E., Scott, L. H., & Wigglesworth, M. J. (2021). A Perspective on Synthetic Biology in Drug Discovery and Development—Current Impact and Future Opportunities. SLAS Discovery, 26(5), 581–603. https://doi.org/10.1177/24725552211000669
- Hicks, M., Bachmann, T. T., & Wang, B. (2020). Synthetic Biology Enables Programmable Cell-Based Biosensors. ChemPhysChem, 21(2), 132–144. https://doi.org/10.1002/CPHC.201900739
- Akboğa, D., Saltepe, B., Bozkurt, E. U., & Şeker, U. Ö. Ş. (2022). A Recombinase-Based Genetic Circuit for Heavy Metal Monitoring. Biosensors, 12(2). https://doi.org/10.3390/bios12020122
- Del Valle, I., Fulk, E. M., Kalvapalle, P., Silberg, J. J., Masiello, C. A., & Stadler, L. B. (2021). Translating New Synthetic Biology Advances for Biosensing Into the Earth and Environmental Sciences. Frontiers in Microbiology, 11, 3513. https://doi.org/10.3389/fmicb.2020.618373
- Marchand, A., Van Hall-Beauvais, A. K., & Correia, B. E. (2022). Computational design of novel protein–protein interactions – An overview on methodological approaches and applications. Current Opinion in Structural Biology, 74, 102370. https://doi.org/10.1016/J.SBI.2022.102370
- Rosa, S., Bertaso, C., Pesaresi, P., Masiero, S., & Tagliani, A. (2021). Synthetic Protein Circuits and Devices Based on Reversible Protein-Protein Interactions: An Overview. Life, 11(11). https://doi.org/10.3390/LIFE11111171
- Mishra, D., Bepler, T., Teague, B., Berger, B., Broach, J., & Weiss, R. (2021). An engineered protein-phosphorylation toggle network with implications for endogenous network discovery. Science (New York, N.Y.), 373(6550). https://doi.org/10.1126/SCIENCE.AAV0780
- Brooks, S. M., & Alper, H. S. (2021). Applications, challenges, and needs for employing synthetic biology beyond the lab. Nature Communications 2021 12:1, 12(1), 1–16. https://doi.org/10.1038/s41467-021-21740-0
- Sato, W., Zajkowski, T., Moser, F., & Adamala, K. P. (2022). Synthetic cells in biomedical applications. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 14(2), e1761. https://doi.org/10.1002/WNAN.1761
- Wagner, A. M., Eto, H., Joseph, A., Kohyama, S., Haraszti, T., Zamora, R. A., Vorobii, M., Giannotti, M. I., Schwille, P., Rodriguez-Emmenegger, C., Wagner, A. M., Joseph, A., Haraszti, T., Vorobii, M., Rodriguez-Emmenegger, C., Eto, H., Kohyama, S., & Schwille, P. (2022). Dendrimersome Synthetic Cells Harbor Cell Division Machinery of Bacteria. Advanced Materials, 34(28), 2202364. https://doi.org/10.1002/ADMA.202202364
- Zhan, P., Jahnke, K., Liu, N., & Göpfrich, K. (2022). Functional DNA-based cytoskeletons for synthetic cells. Nature Chemistry 2022 14:8, 14(8), 958–963. https://doi.org/10.1038/s41557-022-00945-w
- Wiesauer, M., & Knör, G. (2019). Towards artificial cells for biomedical applications. Biomedical Research and Clinical Practice, 4(3). https://doi.org/10.15761/BRCP.1000189

