Elif AtayT., Elif DuymazE.
Living therapeutics is the name of living organisms conceived to treat, prevent a disease, or maintain the current situation1. A living therapeutic can also be designed to produce and represent other therapeutics asin its original place2. Synthetic biology, which has a great contribution to living therapeutics’ design, production, and improvement, is a branch of science that aims at recreating the existing components in living organisms or producing new pieces for determined function3. It provides the production of precious chemicals, participates in the construction of biofactories, where biofuels will be produced, and plays a role in designing and constructing artificial biological systems that are programmed for various purposes, such as enhancing human health and the environment through an engineering perspective (Figure 1)4. In this review, it will be mentioned about multidisciplinary approaches and methods that are used for microorganism-based biomedical applications.
Relationship Between Synthetic Biology and Living Therapeutics
Increasing literature on the molecular basis of diseases in parallel with the development of DNA technologies that are essential and efficient for combating microbial genomes and silence them, enables the engineering of living therapeutics which are specifically designed for human diseases’ treatments5. With advances in synthetic biology, improvements, which are done by scientists, and the use of cells that are equipped with synthetic gene circuits are considered a better alternative than small molecules or biological agents6. One of the main reasons for this tendency is the occurrence of large-scale outbreaks throughout human history7. The biggest problem in forming needed strategies for communicable diseases’ occurrence is the development of diagnosis and treatment pace that cannot keep up with the speed of disease spread8. At this point, synthetic biology stands out in terms of the efficacy and speed in the control of communicable diseases7-9.
For instance, designing microorganisms as synthetic biology-based living therapeutics is considered an effective method for diagnosis and treatment of many health problems. Additionally, it is also possible to utilize specific features of these microorganisms for biosafety10.
Relationship Between Bacteria and Humans
It is thought that the number of colonized microorganisms is ten times more than the total number of our cells11. The bacteria, which are generally seen as causative agents of communicable diseases, but which we host, and which are mostly non-pathogenic, are fundamental elements for humans’ health. Bacteria are actively applied in the treatment of human diseases for more than a century. These applications are carried out with natural and pure samples obtained from healthy microbiotas of people, and they are used as “probiotics” by us5. Probiotics are defined as living microorganisms that provide health benefits to hosts when used in sufficient and appropriate amounts12.
Over time, with the emergence of the ability to manipulate and make changes to human biology, bacteria can be used as ‘probiotics’ to fight against diseases. In other words, after appropriate engineering processes, they might also be considered living therapeutics thanks to synthetic biology with the emergence of the ability to manipulate and make changes to human biology, they can use bacteria as “probiotics” to fight against diseases; in other words, after the appropriate engineering processes, thought of they might also13-15.
Necessary Studies for Identification and Treatment of Communicable Diseases
The first step is selecting the convenient bacteria which is going to be engineered. Since the dysregulated interaction between the host organism and bacteria has correlated with diseases such as obesity, cancer, and inflammatory bowel disease (IBD) has been established; commensal (common) or non-common, but; it is significantly important to choose attenuated bacteria species to avoid triggering any of immune response in the body16,17.
The second step is designing an intermediary genetic circuit that will alter the selected bacterium as therapeutic according to the appropriate aim, and this tool should be applied in situ3. As a therapeutic agent; different toxins, peptides, or proteins can be selected to block the growth of infectious agent or eliminating them completely3,18.
The third and the last step is application. It has critical importance to check the bacteria after introducing them into the body for a therapeutic purpose3. Once the goal is reached, the bacteria should be destroyed in the body. Two modalities exist for these:
- If the bacteria are designed as not being antibiotic-resistant, elimination of bacteria in the body can be provided by applying antibiotics at the end of the therapy3.
- At the designing stage, destruction of the bacteria population might be provided when critical population concentration is reached via integration of a lysis circuit that is synchronized to genomes of bacteria3.
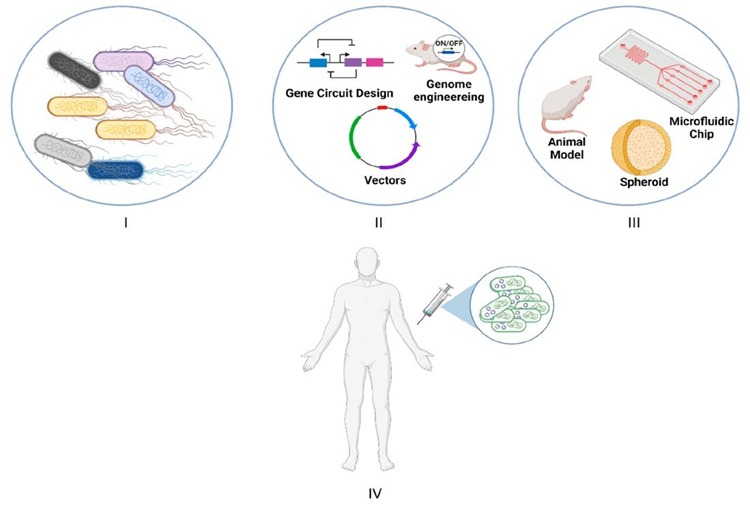
Figure 1. Schematic illustration of the flowchart in bacteria-based living therapeutics3. (I) Selection of bacteria that will be used. (II) Bacteria that is made therapeutic by engineering (Genome engineering that has optimized and efficient genetic circuits and/or plasmid can be applied.) (III) In vivo testing the systems with the use of in vitro or model organisms via mammalian cell culture, microfluidic chips, and spheroids (spheroidal objects). (IV) Human trials.
In therapeutic methods, there are three common therapy types known as microbiome therapeutics:
- Additive Therapy: It takes place by the attachment of microbial strains/consortiums19,20.
- Subtractive Therapy: It aims to eliminate specific, known, and fatal pathogens that are causing disease19-21.
- Modulatory Therapy: It is used for manipulating or altering the interaction between host and microbiome for specific functions utilizing particular death agents19,20.
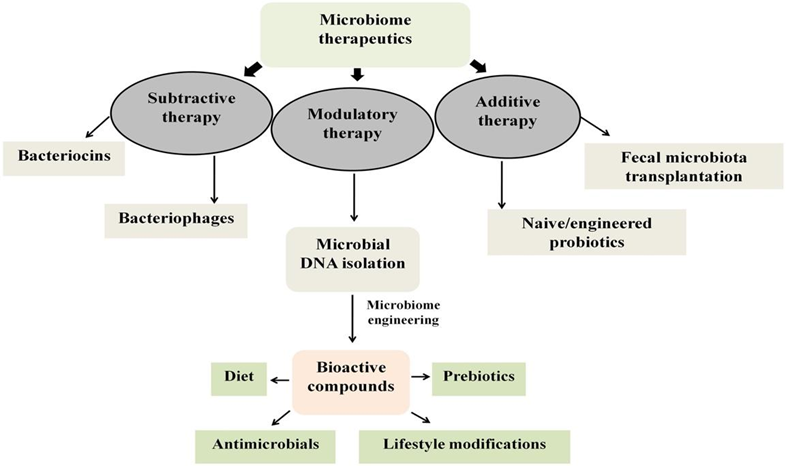
Figure 2. Overview of strategies used as microbiome therapeutics19.
Examples of Microbiota Therapeutics
It is known that humans’ gut microbiota is also an important factor for human health conditions against metabolic diseases as well as the immune-mediated and pathogen-dependent diseases22. Besides the healthy microbiome of a person plays a significant role in disease combat, the occurrence of dysbiosis might lead to plenty of diseases’ onset19.
– Christensenella sp.: This bacterium has been found to be beneficial for psychological disorders such as depression and anxiety, which are often associated with behavioral disorder19.
– Akkermansia muciniphila: This bacterium is known to alleviate metabolic deficiencies, collaborate with the use of metformin in cancer treatment, and protect against atherosclerosis by reducing inflammation and improving intestinal permeability23,24.
– Lactobacillus johnsonii: This bacterium has been found to provide protection against cancer25.
Lactic Acid Bacteria-Based Living Therapeutics
The human microbiota refers to the ecosystem of microorganisms that live in the human body26. Microbiota acts as a well-tolerant buffer area that has broad and high interaction between host and external environment26. With the studies on microbiomes in human bodies after the advent of Next-Generation Sequencing (NGS), and many more, it is revealed that humans are not only hosts but also active members of a functioning community27. Human health and development are directly relevant to microbiota health27-29. Since the studies and conclusions, which supports this sentence, are increasing day by day, the factor that microbiota is a health and disease regulator is added to its definition26. According to the studies carried out to exemplify this, intestinal microbiota is associated with atopy, which is a particular allergic sensitization, asthma, cardiovascular diseases, neuropsychiatric disorders, cancer, pediatric diseases, and senescence1,27-30. Numerous studies have focused on improving the microbiota-host relationship by restoring internal environment homeostasis through the use of nutrition or substances called ‘probiotics’ and ‘prebiotics’ in the treatment of these diseases27,29,31. Over time, Lactic Acid bacteria (LAB), which is from Lactobacillales order and used for nourishment and agricultural processing, has passed into the center of these studies26. It is observed that prebiotic/probiotic supplement, which is also used with the adjuvant approach (promoter of fundamental treatment), is more effective in elimination of Helicobakter pilori or Clostridium difficile infections when it is used with antibiotics compared to the mere utilization of antibiotics27,31.
LABs, whose deficiency plays a critical role in the occurrence of diseases and can be utilized for certain biosynthetic processes in disease treatment, also directly interacting with skin health. Recently, many researchers examined the interaction between LABs and skin health32. The consensus which occurs as a result of different studies is that probiotics have positive impacts on skin health. In a study, it is suggested that bacteriotherapy, which is made by oral way via the use of probiotics, emerges beneficial effects on treatments of certain dermatologic diseases32,33. Probiotics help maintain skin homeostasis by providing protection not only against diseases but, also against UV light32.
Traditional Treatments and Living Therapeutics
Despite limitations of traditional treatments that are applied in infectious diseases and cancer such as pathogen resistance, chemotherapy resistance, or inadequate response to drugs, respectively, living therapeutics have the potential to play a key role in eliminating difficulties which are faced during the existing treatment approaches and increasing the success of treatment since they can be programmed for complex duties4,19.
The reprogrammability of living therapeutics using different sensors and actuators is faster, more efficient, flexible, and cost-effective compared to traditional therapies. It offers customization and vulnerability, ultimately saving time and creating more positive outcomes for the health of the human population3,4,19.
It is possible to correlate any of the existing disorders directly with the physiology of the patient, since the data obtained from patients’ bodies are real-time4. Another problem formed by traditional modalities in combating contagious and especially viral diseases is the testing methods are prone to lack the substantial quantity of time and accuracy3,34,35.
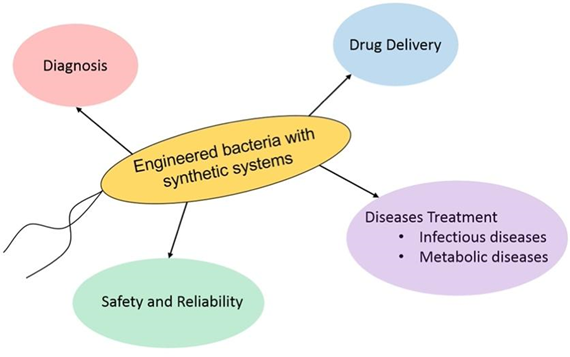
Figure 4. Scheme for explaining which therapeutic purposes are applied for synthetic bacteria4.
An On-Going Treatment Modality from Traditional Methods: Antibiotics
Salvarsan, which was the first antibiotic, began to be used in 191036. Since penicillin was discovered by English doctor Sir Alexander Fleming in 1928, antibiotics have been outstanding options for the treatment of many bacterial infectious diseases3,37. The discovery of penicillin became an onset of the golden age in terms of searching for natural and other types of antibiotics, yet this era had been continued until the middle of 1950s, when the research and developments were depleted and weakened3,36,38. From their discovery until now, antibiotics have not only drastically changed modern medicine, but also significantly reduced the number of deaths caused by infections in a way that prolonging the average human lifespan approximately 23 years in 100 years3,39. On the other hand, the methods developed against the bacteria which have increasing evolution speed and antibiotic resistance are slower than their speed of evolution3. Nevertheless, it is a hopeful approach to improve functional scale of probiotics against an increase in antibiotic-resistant pathogens and slower development of new antibiotics40.
Living therapeutics, which have been used for centuries with the same purpose as today but through different and more primitive methods, represent a multidisciplinary scientific field. This field holds great promise for the future in terms of disease diagnosis and treatment. It continually evolves and stays up to date with the advancements in technology, biology, engineering, and many other fields, making it a field of ongoing development.
References:
- Charbonneau, M. R., Isabella, V. M., Li, N., & Kurtz, C. B. (2020). Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nature communications, 11(1), 1738. https://doi.org/10.1038/s41467-020-15508-1
- Pedrolli, D. B., Ribeiro, N. V., Squizato, P. N., de Jesus, V. N., Cozetto, D. A., & Team AQA Unesp at iGEM 2017 (2019). Engineering Microbial Living Therapeutics: The Synthetic Biology Toolbox. Trends in biotechnology, 37(1), 100–115. https://doi.org/10.1016/j.tibtech.2018.09.00
- Khan, A., Ostaku, J., Aras, E., & Safak Seker, U. O. (2022). Combating Infectious Diseases with Synthetic Biology. ACS synthetic biology, 11(2), 528–537. https://doi.org/10.1021/acssynbio.1c00576
- Vo, P., Lee, H. M., & Na, D. (2019). Synthetic Bacteria for Therapeutics. Journal of microbiology and biotechnology, 29(6), 845–855. https://doi.org/10.4014/jmb.1904.04016
- Piñero-Lambea, C., Ruano-Gallego, D., & Fernández, L. Á. (2015). Engineered bacteria as therapeutic agents. Current opinion in biotechnology, 35, 94–102. https://doi.org/10.1016/j.copbio.2015.05.00
- Cubillos-Ruiz, A., Guo, T., Sokolovska, A., Miller, P. F., Collins, J. J., Lu, T. K., & Lora, J. M. (2021). Engineering living therapeutics with synthetic biology. Nature reviews. Drug discovery, 20(12), 941–960. https://doi.org/10.1038/s41573-021-00285-3
- Piret, J., & Boivin, G. (2021). Pandemics Throughout History. Frontiers in microbiology, 11, 631736. https://doi.org/10.3389/fmicb.2020.631736
- Vickers, C. E., & Freemont, P. S. (2022). Pandemic preparedness: synthetic biology and publicly funded biofoundries can rapidly accelerate response time. Nature communications, 13(1), 453. https://doi.org/10.1038/s41467-022-28103-3
- Gordillo Altamirano, F. L., & Barr, J. J. (2019). Phage Therapy in the Postantibiotic Era. Clinical microbiology reviews, 32(2), e00066-18. https://doi.org/10.1128/CMR.00066-18
- Kelly, V. W., Liang, B. K., & Sirk, S. J. (2020). Living Therapeutics: The Next Frontier of Precision Medicine. ACS synthetic biology, 9(12), 3184–3201. https://doi.org/10.1021/acssynbio.0c00444
- Xu, J., Mahowald, M. A., Ley, R. E., Lozupone, C. A., Hamady, M., Martens, E. C., Henrissat, B., Coutinho, P. M., Minx, P., Latreille, P., Cordum, H., Van Brunt, A., Kim, K., Fulton, R. S., Fulton, L. A., Clifton, S. W., Wilson, R. K., Knight, R. D., & Gordon, J. I. (2007). Evolution of symbiotic bacteria in the distal human intestine. PLoS biology, 5(7), e156. https://doi.org/10.1371/journal.pbio.0050156
- Jensen, H., Grimmer, S., Naterstad, K., & Axelsson, L. (2012). In vitro testing of commercial and potential probiotic lactic acid bacteria. International journal of food microbiology, 153(1-2), 216–222. https://doi.org/10.1016/j.ijfoodmicro.2011.11.020
- Dou, J., & Bennett, M. R. (2018). Synthetic Biology and the Gut Microbiome. Biotechnology journal, 13(5), e1700159. https://doi.org/10.1002/biot.201700159
- Cruz, K., Enekegho, L. O., & Stuart, D. T. (2022). Bioengineered Probiotics: Synthetic Biology Can Provide Live Cell Therapeutics for the Treatment of Foodborne Diseases. Frontiers in bioengineering and biotechnology, 10, 890479. https://doi.org/10.3389/fbioe.2022.890479
- Stavropoulou, E., & Bezirtzoglou, E. (2020). Probiotics in Medicine: A Long Debate. Frontiers in immunology, 11, 2192. https://doi.org/10.3389/fimmu.2020.02192
- van der Lelie, D., Oka, A., Taghavi, S., Umeno, J., Fan, T. J., Merrell, K. E., Watson, S. D., Ouellette, L., Liu, B., Awoniyi, M., Lai, Y., Chi, L., Lu, K., Henry, C. S., & Sartor, R. B. (2021). Rationally designed bacterial consortia to treat chronic immune-mediated colitis and restore intestinal homeostasis. Nature communications, 12(1), 3105. https://doi.org/10.1038/s41467-021-23460-x
- Tan, Y., Shen, J., Si, T., Ho, C. L., Li, Y., & Dai, L. (2020). Engineered Live Biotherapeutics: Progress and Challenges. Biotechnology journal, 15(10), e2000155. https://doi.org/10.1002/biot.202000155
- Omer, R., Mohsin, M. Z., Mohsin, A., Mushtaq, B. S., Huang, X., Guo, M., Zhuang, Y., & Huang, J. (2022). Engineered Bacteria-Based Living Materials for Biotherapeutic Applications. Frontiers in bioengineering and biotechnology, 10, 870675. https://doi.org/10.3389/fbioe.2022.870675
- Yadav, M., & Chauhan, N. S. (2021). Microbiome therapeutics: exploring the present scenario and challenges. Gastroenterology report, 10, goab046. https://doi.org/10.1093/gastro/goab046
- Singhvi, G., Girdhar, V., Patil, S., Gupta, G., Hansbro, P. M., & Dua, K. (2018). Microbiome as therapeutics in vesicular delivery. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 104, 738–741. https://doi.org/10.1016/j.biopha.2018.05.099
- Mimee, M., Citorik, R. J., & Lu, T. K. (2016). Microbiome therapeutics – Advances and challenges. Advanced drug delivery reviews, 105(Pt A), 44–54. https://doi.org/10.1016/j.addr.2016.04.032
- Waters, J. L., & Ley, R. E. (2019). The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC biology, 17(1), 83. https://doi.org/10.1186/s12915-019-0699-4
- De la Cuesta-Zuluaga, J., Mueller, N. T., Corrales-Agudelo, V., Velásquez-Mejía, E. P., Carmona, J. A., Abad, J. M., & Escobar, J. S. (2017). Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes care, 40(1), 54–62. https://doi.org/10.2337/dc16-1324
- Li, J., Lin, S., Vanhoutte, P. M., Woo, C. W., & Xu, A. (2016). Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation, 133(24), 2434–2446. https://doi.org/10.1161/CIRCULATIONAHA.115.019645
- Cheema, A. K., Maier, I., Dowdy, T., Wang, Y., Singh, R., Ruegger, P. M., Borneman, J., Fornace, A. J., Jr, & Schiestl, R. H. (2016). Chemopreventive Metabolites Are Correlated with a Change in Intestinal Microbiota Measured in A-T Mice and Decreased Carcinogenesis. PloS one, 11(4), e0151190. https://doi.org/10.1371/journal.pone.0151190
- Ozdemir, T., Fedorec, A., Danino, T., & Barnes, C. P. (2018). Synthetic Biology and Engineered Live Biotherapeutics: Toward Increasing System Complexity. Cell systems, 7(1), 5–16. https://doi.org/10.1016/j.cels.2018.06.008
- Mays, Z. J., & Nair, N. U. (2018). Synthetic biology in probiotic lactic acid bacteria: At the frontier of living therapeutics. Current opinion in biotechnology, 53, 224–231. https://doi.org/10.1016/j.copbio.2018.01.028
- Valdes, A. M., Walter, J., Segal, E., & Spector, T. D. (2018). Role of the gut microbiota in nutrition and health. BMJ (Clinical research ed.), 361, k2179. https://doi.org/10.1136/bmj.k2179
- Ogunrinola, G. A., Oyewale, J. O., Oshamika, O. O., & Olasehinde, G. I. (2020). The Human Microbiome and Its Impacts on Health. International journal of microbiology, 2020, 8045646. https://doi.org/10.1155/2020/8045646
- Altveş, S., Yildiz, H. K., & Vural, H. C. (2020). Interaction of the microbiota with the human body in health and diseases. Bioscience of microbiota, food and health, 39(2), 23–32. https://doi.org/10.12938/bmfh.19-023
- Del Rio, B., Redruello, B., Fernandez, M., Martin, MC, Ladero, V., & Alvarez, MA (2019). Lactic Acid Bacteria as a Live Delivery System for the in situ Production of Nanobodies in the Human Gastrointestinal Tract Frontiers in Microbiology, 9. doi:10.3389/fmicb.2018.03179
- Jeong, J. H., Lee, C. Y., & Chung, D. K. (2016). Probiotic Lactic Acid Bacteria and Skin Health. Critical reviews in food science and nutrition, 56(14), 2331–2337. https://doi.org/10.1080/10408398.2013.834874
- Wang, L., Yu, T., Zhu, Y., Luo, Y., Dong, F., Lin, X., Zhao, W., He, Z., Hu, S., & Dong, Z. (2022). Amplicon-based sequencing and co-occurence network analysis reveals notable differences of microbial community structure in healthy and dandruff scalps. BMC genomics, 23(1), 312. https://doi.org/10.1186/s12864-022-08534-4
- Tsao, Y. T., Tsai, Y. H., Liao, W. T., Shen, C. J., Shen, C. F., & Cheng, C. M. (2020). Differential Markers of Bacterial and Viral Infections in Children for Point-of-Care Testing. Trends in molecular medicine, 26(12), 1118–1132. https://doi.org/10.1016/j.molmed.2020.09.004
- Tribolet, L., Kerr, E., Cowled, C., Bean, A., Stewart, C. R., Dearnley, M., & Farr, R. J. (2020). MicroRNA Biomarkers for Infectious Diseases: From Basic Research to Biosensing. Frontiers in microbiology, 11, 1197. https://doi.org/10.3389/fmicb.2020.01197
- Hutchings, M. I., Truman, A. W., & Wilkinson, B. (2019). Antibiotics: past, present and future. Current opinion in microbiology, 51, 72–80. https://doi.org/10.1016/j.mib.2019.10.008
- Uddin, T. M., Chakraborty, A. J., Khusro, A., Zidan, B., Mitra, S., Emran, T. B., Dhama, K., Ripon, M., Gajdács, M., Sahibzada, M., Hossain, M. J., & Koirala, N. (2021). Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. Journal of infection and public health, 14(12), 1750–1766. https://doi.org/10.1016/j.jiph.2021.10.020
- Ribeiro da Cunha, B., Fonseca, L. P., & Calado, C. (2019). Antibiotic Discovery: Where Have We Come from, Where Do We Go?. Antibiotics (Basel, Switzerland), 8(2), 45. https://doi.org/10.3390/antibiotics8020045
- Durand, G. A., Raoult, D., & Dubourg, G. (2019). Antibiotic discovery: history, methods and perspectives. International journal of antimicrobial agents, 53(4), 371–382. https://doi.org/10.1016/j.ijantimicag.2018.11.010
- Singh, B., Mal, G., & Marotta, F. (2017). Designer Probiotics: Paving the Way to Living Therapeutics. Trends in biotechnology, 35(8), 679–682. https://doi.org/10.1016/j.tibtech.2017.04.001

